ISPOR 2025 is now in about two weeks and in this second post about sessions to look for, I’ll talk about regulations and pricing (this is part of a series: last week, I wrote about Modelling; next week, I will write about AI).

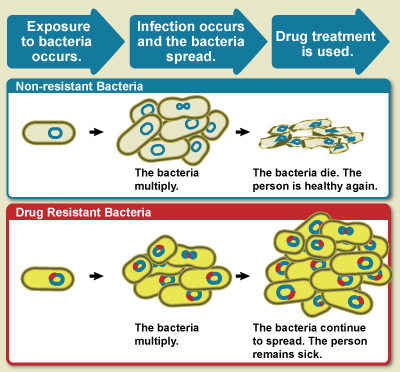
As the main edition of ISPOR is held in North America, the Inflation Reduction Act (IRA) will naturally attract considerable attention. It will start with the very first Plenary Session, promising to explore the impact of price limits on innovation and provide real-world examples.
The lessons learnt or (unintended) consequences-type of sessions are in vogue, with sessions like “Year 1 learnings“, “Implications for Providers“, “Did the IRA spook the industry” (from the perspective of rare diseases), “Unintended consequences“, and a discussion “Beyond Drug Negotiation“.
Here is one session to remind you that timing is essential … The IRA was introduced in 2022, under US President Biden, but the first Maximum Fair Prices will be implemented in 2026, under US President Trump. The latter has already introduced sweeping changes in the pharmaceutical research landscape in the US. But I found one interesting issue panel somewhat prophetic: “IRA Under Trump: What Is Next?“. Given the timelines for panel submission for ISPOR (before the 2024 elections), I have so many scenarios in my head about the authors trying to write the abstract broad enough to encompass potential futures while navigating the possible sensitivity of the situation, even before it happens. It will be an interesting panel to attend …
A panel also takes another perspective, wondering if the IRA became the US HTA. From the abstract, I understand the authors’ perspective, but I don’t agree: although the IRA will impact millions of Americans, it still lacks a direct impact on commercial plans. But it’s obviously a broader discussion than a terse comment in a blog post.

It will also be the first ISPOR conference after the European Joint Clinical Assessment (JCA) process started in Europe. Therefore, it is a bit early to draw conclusions and look at lessons learnt. However, two interesting sessions will examine it from the outside: one will examine the global (i.e. ex-EU) impact of JCA, and another will consider JCA as an enabler of cross-border collaborations.
Finally, because I recently contributed to projects supporting investors with health economics tools and assessments, I will be interested in the Input/Output Modelling panel: it will look at the broader impact of investments in health and pharmaceutical products. Their abstract reminded me of some early work published by a former boss on the societal impact of vaccination (I wonder if vaccines will be mentioned, by the way). The last workshop (the last one mentioned here) is titled “HEOR meets investing“, and it is precisely what we recently did: early health economics modelling can greatly help secure investments by reassuring about the potential cost-effectiveness of a drug, and justifying studies to fill crucial input data gaps.
I missed some panels in this short helicopter view. Do you have any other suggestions?
Next week, I will look at AI’s potential progress in health economics. Stay tuned!